Originally published 27 December 1999
What was the most important science story of the century?
Computers, DNA, and nuclear physics are the obvious choices. But computers and DNA are mostly stories of the second half of the century, and both stories still have a long way to run. Nuclear physics came in with the century, it culminated at the century’s middle, and now, at century’s end — if we are lucky — the story is winding down.
The nuclear physics story can be posed as a question: What makes the stars shine? No question can be more fundamental. Why is the universe bright with light? What is the source of the sun’s energy, upon which all life on Earth depends?
One hundred years ago, no one had an answer. Geologists and biologists had determined that life on the Earth was hundreds of millions, perhaps billions of years old, but no source of energy known at that time could keep the sun burning steadily for so long.
One evening in 1902, Marie and Pierre Curie put their children to bed and slipped away from their house in Paris. They walked through the still busy streets to their laboratory, where for four years Marie had labored mightily to extract a tenth of a gram of a new element from tons of pitchblende ore from Czechoslovakia purchased with their meager savings.
As Marie had labored, wife and husband had wondered what the element she sought would look like. Would it be gray, black, shiny, dull? The answer was unlike anything they might have guessed. As they stood in the darkened lab (“Don’t light the lamps,” Marie whispered.) the precious element glowed with a spontaneous light in its glass containers. “Look, look,” Marie murmured ecstatically, as she gazed at the pale-blue glimmering.
Marie Curie did not know the source of the mysterious light that came from of the stuff she named radium. She could not have dreamed that the light from her glass vials had the same source as the light of the stars.
The next piece of the puzzle fell into place in 1915. Albert Einstein, in Berlin, was working on new ideas about gravity, space, and time. For five weeks he was consumed in a frenzy of creativity, skipping meals and working far into the night. When he did eat, he cooked eggs and soup together in the same pot to save time. At last he had before him an elegant mathematical theory — the theory of general relativity — what some have called “the supreme intellectual achievement of the human species.”
Among other things, the theory predicted the equivalence of matter and converted into the other. This would soon be recognized as the source of Marie Curie’s pale light: In the spontaneous fissioning (breaking apart) of radium nuclei, mass is converted into pure luminous energy.
The atomic nucleus was intensely studied by physicists during the 1920s and 1930s. By the spring of 1939, it was recognized that when the nucleus of uranium-235 fissions, neutrons are emitted that can collide with other uranium nuclei and cause them to split. Under the proper circumstances, a chain reaction appeared to be possible — a vast amount of energy suddenly released from a small amount of matter.
American physicists imposed upon Einstein to write a letter to President Franklin Delano Roosevelt describing recent fission studies of Enrico Fermi and Leo Szilard. “It is conceivable,” Einstein wrote, “that extremely powerful bombs of this type may be constructed.” Roosevelt reacted decisively. The Manhattan Project was initiated to produce sufficient quantities of uranium to make a nuclear weapon.
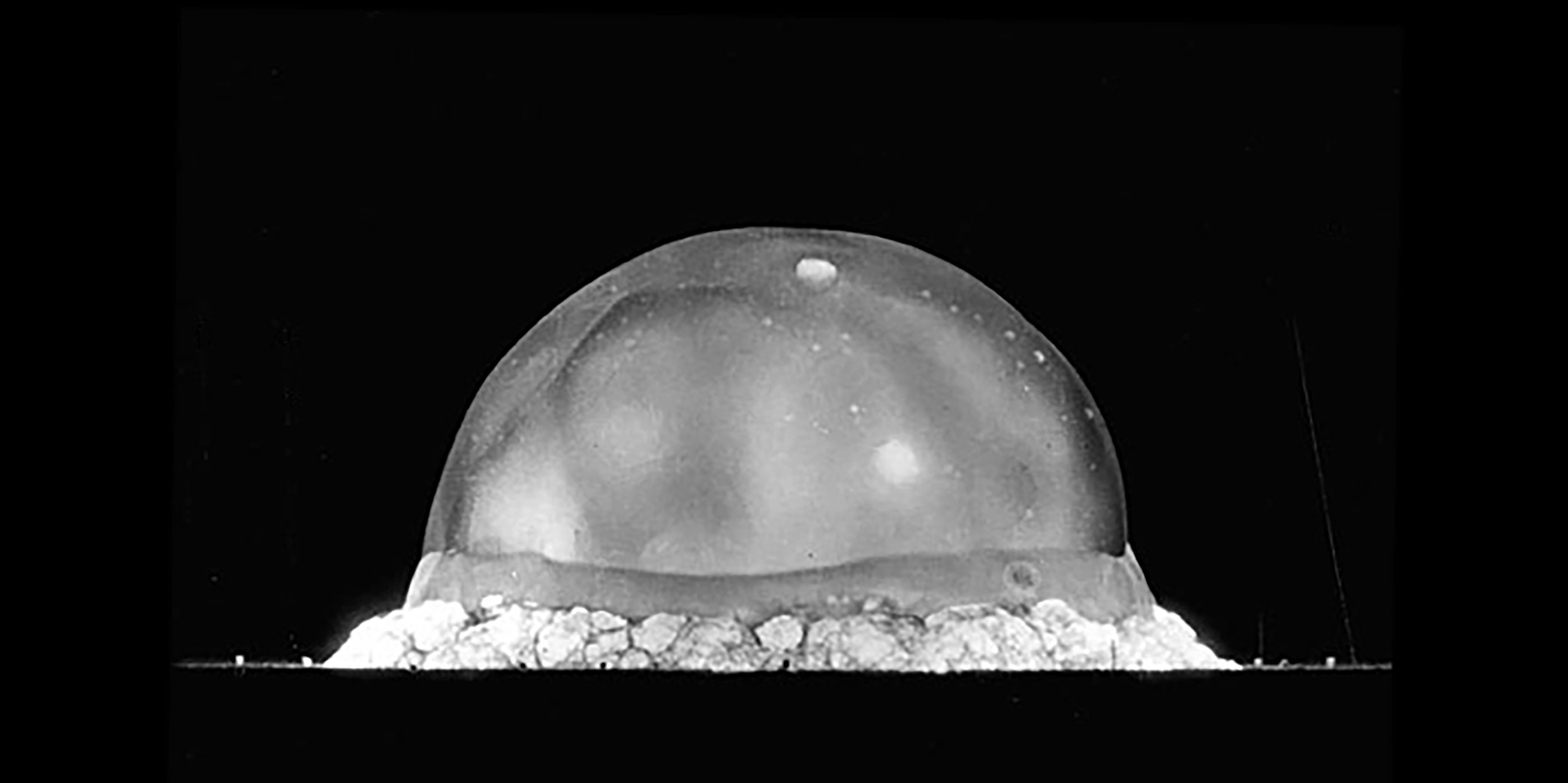
At dawn on the morning of July 16, 1945, in the New Mexico desert, the Nuclear Age was born. Marie Curie’s pale glow was turned into a blaze of light like a thousand suns. A seething mushroom cloud rose heavenward from the desert floor. Within months, two great cities had been obliterated and a global war ended.
Heavy elements, like radium and uranium, release energy when they fission. Light elements release energy when they fuse, although this process is not spontaneous; for example, exceedingly high temperatures are required to make hydrogen fuse into helium. This is what happens at the center of the sun — and other stars — where the squeeze of gravity produces temperatures of tens of millions of degrees.
Fission bombs can provide sunlike temperatures here on Earth. It wasn’t long before physicists used a fission bomb to fuse hydrogen. On November 1, 1952, a fusion bomb was exploded at Eniwetok atoll in the Pacific Ocean that was many times more powerful than the weapons that obliterated Hiroshima and Nagasaki.
The mushroom clouds of those terrible explosions overarch the century’s middle, casting a retrospective shadow over exciting intellectual developments of the first half of the century, and scaring us half to death throughout the second half. The latter gloom was somewhat ameliorated by the promise of peaceable uses of nuclear energy. “The Atom: Our Obedient Servant” was the title of one over-optimistic magazine article in the 50s.
The bright promise of harnessing the atomic nucleus to provide cheap, inexhaustible energy mostly evaporated with the accidents at Three-Mile Island and Chernobyl. And the collapse of the Soviet Union diminished the likelihood of global thermonuclear war. All in all, nuclear physics is not so much on our minds as it was a half-century ago.
The Nuclear Age left a frightening legacy. Marie Curie died of radiation poisoning, and the planet is poisoned by radioactive waste.
On the up side, we now understand what makes the stars burn, and why life on Earth is possible. The “supreme intellectual achievement of the human species” has explained the origin and evolution of the universe. “Let there be light,” says the Bible. It was a nuclear light. Our century’s light. Wonderful and terrible.