Originally published 22 October 2002
Malaria kills more than a million people a year, most of them children under the age of 5 in sub-Saharan Africa. Hundreds of millions of people have the disease, in varying degrees of severity.
The name malaria means “bad air.” The dank air of marshy land was long thought to cause the disease. In the 19th century, mosquitoes were fingered as the culprit.
But mosquitoes are no more the cause of malaria than bad air. The agent of infection is a nasty little parasite named Plasmodium falciparum that requires two other species to complete its life cycle — the mosquito Anopheles gambiae, and the primate Homo sapiens.
That’s us.
Plasmodium doesn’t bear us any grudge. From the parasite’s point of view, we are a useful (and necessary) blob of meat in which to breed.
Here’s how it works.
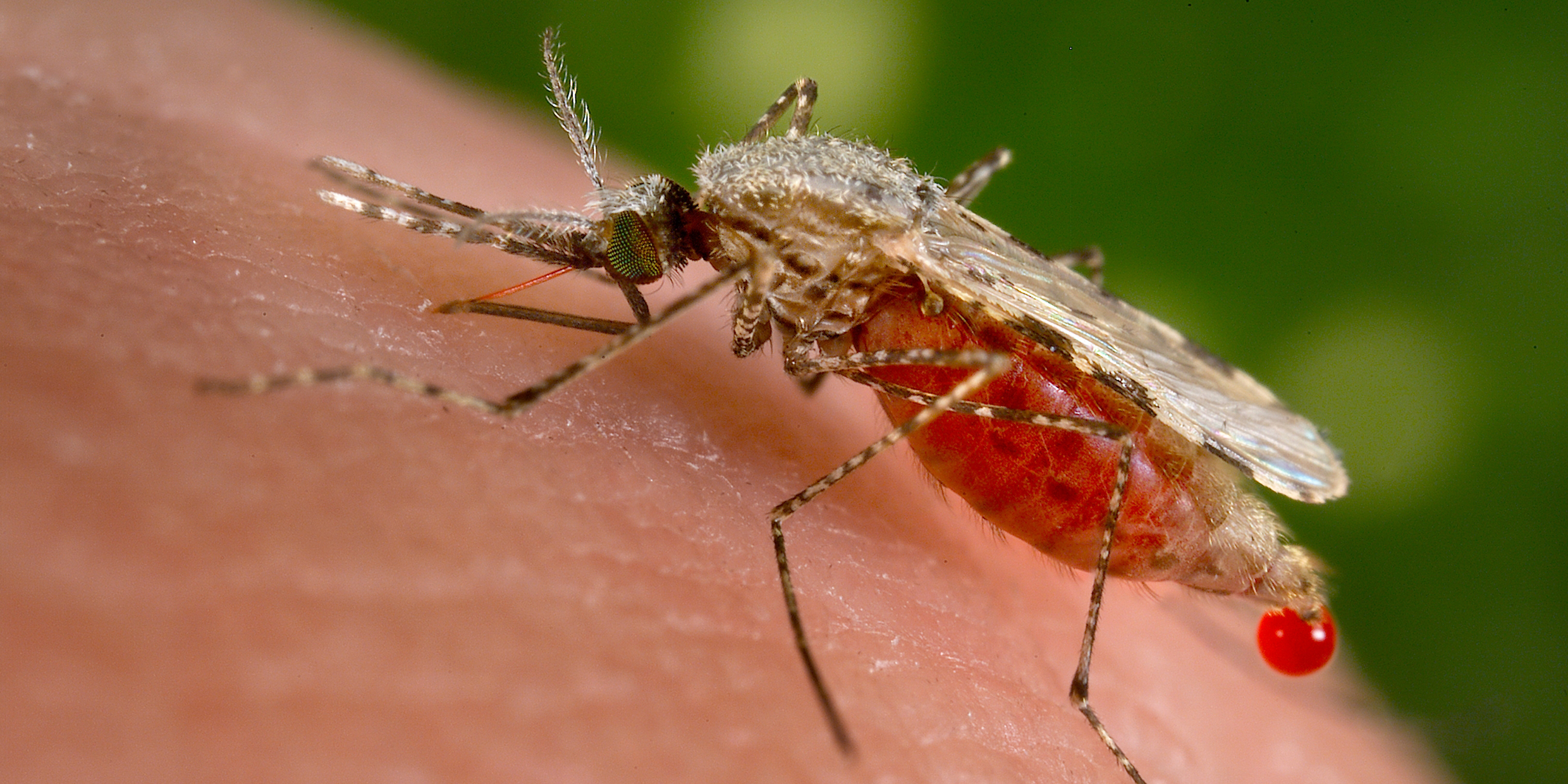
An infected female Anopheles mosquito bites a human and injects parasites into the victim’s bloodstream.
The parasites make their way to the liver and multiply.
The teeming parasites explode from the liver cells back into the bloodstream, where they invade red blood cells and multiply again. They fill the cells to bursting, then invade more red blood cells. Again. And again. The malaria victim sustain bouts of fever with each cycle of cell infection.
If an uninfected female Anopheles mosquito bites an infected human, she sucks up parasites with her blood meal. These multiply in her stomach wall, then make their way to her salivary glands, ready for transfer to another human.
And so it goes, around and around — human liver, human blood, mosquito stomach, mosquito salivary gland. Each stage of Plasmodium’s life cycle involves a specialized form of the parasite. No other hosts will do but Homo sapiens and Anopheles gambiae.
There’s no moral dimension to the story, no clash of good and evil. Plasmodium is just making a living in the way that is programmed into its genes. The mosquito, too, just wants her dinner. And Homo sapiens? Well, we’ve certainly made a lot of ourselves. It would be surprising if other creatures did not make use of so much readily available protein.
And when you think about it, the story is all about proteins. Proteins that let the parasite latch into human liver cells, and into red blood cells. Proteins that let the parasite attach itself to the mosquito’s stomach wall, and then allow it to make its way to the mosquito’s salivary gland. Twisty protein molecules that meet and fit together like lock and key. A gorgeous dance of geometry.
And where do all these specialized proteins come from? The instructions for making them are all there, written in a four-letter chemical code on Plasmodium’s DNA.
Which has now been sequenced.
You can see a beautiful color-coded map of the malaria parasite’s DNA in the October 3 2002 issue of the journal Nature. An estimated 5,300 genes on 14 chromosomes, 23 million steps on the spiral staircase of the DNA’s double helix, the score for Plasmodium’s symphony of life, packed into every cell of a creature small enough to hide in a mosquito’s spit.
Here are the instructions for making proteins that control development of the parasite at each stage of its life cycle. Proteins for metabolism, cell reproduction, cell motility, stress response, cell communication, cell death. Proteins that bind and signal and transport and invade. Proteins that control the making of other proteins — untangling the DNA, detecting and repairing errors.
It’s breathtaking. Behind the human tragedy — the dying children, the fits of fever — the chemical music of life plays on.
The genetic code of the Anopheles mosquito has now been sequenced, too; you can see it displayed schematically in the October 4 2002 issue of Science. And, of course, the human genome was revealed last year. So scientists have the genetic codes for all three creatures implicated in malaria.
Knowledge is power. The sequenced genomes of the malaria parasite and the Anopheles mosquito give scientists a leg up in the search for effective drugs and vaccines. They also will facilitate modification of the parasite’s or the mosquito’s genes in ways that might disrupt Plasmodium’s life cycle — ridding the human species of one more mortal plague.
But genetic modification of any creature’s code of life is fraught with dangers for the web of life, many of which are not yet fully understood. Knowledge is power for good or ill. The promises and perils of genetic engineering will test the wisdom of nature’s only moral species.
The best insurance that this new power will be used wisely is a citizenry solidly educated in both the sciences and the arts. The decisions to be made are highly technical, and must be based on scientific knowledge. They are far too important to be left to scientists.