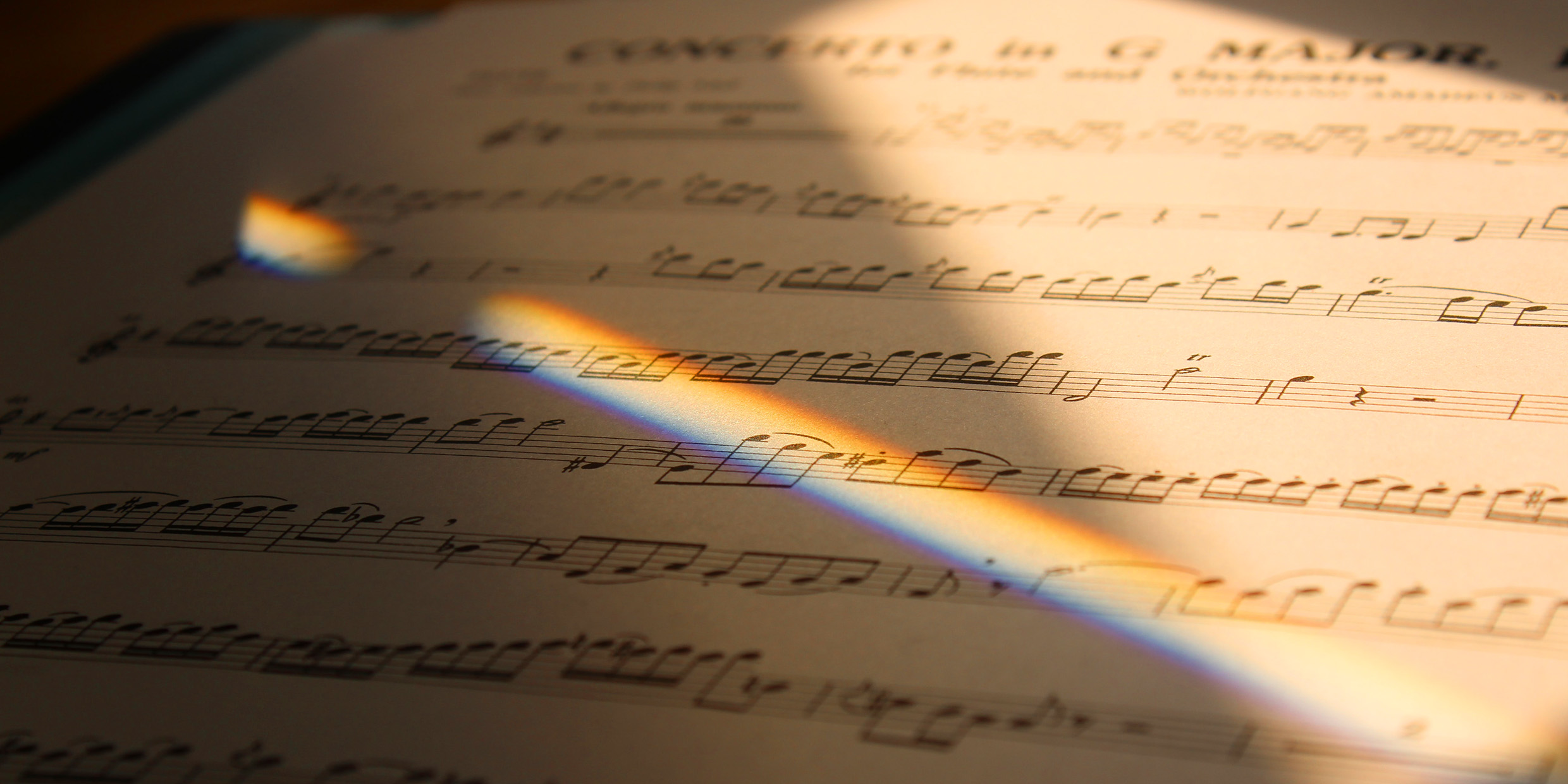Originally published 18 June 2002
If you’ve seen a rainbow, you have seen the colors of starlight.
The sun is a typical star, and the light of other stars also would make earthly rainbows if they weren’t so far away and faint. Astronomers use instruments called spectroscopes to make rainbows of starlight. To the eye, some stars are yellow like the sun, some redder, some bluer, but, through a spectroscope, they all reveal a spectrum of colors, of varying intensities.
Now bear with me through some technical stuff and we’ll get to one of the greatest mysteries of the universe, something so wonderful, so unexpected, we can only shake our heads with awe.
Put sunlight or starlight through a spectroscope and you see something the eye can’t see: The rainbow has narrow gaps, missing colors of light. The missing colors tell us much about stars, including what stars are made of.
Light is emitted and absorbed by atoms. When an atom emits light, atomic electrons drop down in energy. When an atom absorbs light, electrons are boosted in energy.
The crucial thing about atomic electrons is this: They can’t have just any old energies. They can only have certain energies — “quantized” energies.
Each kind of atom has different allowed energy levels, and these determine the colors of light an atom emits or absorbs. Every element has its own spectral “fingerprint” of emitted or absorbed colors.
These are the “missing colors” we see in a solar or stellar spectrum — colors absorbed by atoms in the gassy atmosphere of the star. In the light of stars, we see the same “fingerprints” of absorbed colors we see on Earth. And so we know that the entire universe is made of the same elements as Earth.
But why do atomic electrons have quantized energies? Ah, now we approach the magical heart of my story.
The first person to have a go at finding a rule behind the colorful fingerprints of atoms was the great Danish physicist Niels Bohr in 1913.
Bohr’s rule worked perfectly for hydrogen, the simplest of the elements.
But his rule wasn’t as pretty as physicists would like; it seemed too arbitrary, too unconnected to anything else.
The less arbitrary a theory seems, the happier physicists are.
Ten years after Bohr, the French physicist Louis de Broglie (who was also a musician) showed that Bohr’s rule could be made to seem less arbitrary if atoms were thought of as little vibrating musical instruments rather than, say, tiny billiard balls. The colors that an element emits or absorbs emerge in de Broglie’s theory in much the same way that a violin string of a certain length, density and tension plays only a certain note.
Suddenly, with de Broglie, the lumpish atoms of the ancients began to sing like a Stradivarius.
Finally, in the 1920s, Erwin Schrödinger proposed a single elegant equation, a musical sort of equation, that he said would describe the allowable energy levels of every element — a single equation in which the entire universe becomes a sort of symphony orchestra, with the elements as the various instruments.
Schrödinger’s beautiful equation can be written down in half a line, and just looking at it you would never guess (if you weren’t a mathematician) that it might tell you the missing colors in starlight.
But it does. Unfortunately, the equation is very difficult to solve except for the simplest atoms, such as hydrogen.
When I used to teach these things, there was nothing I liked doing better with students than solving Schrödinger’s equation for the hydrogen atom. It took us several class periods. And, lo and behold, when we got to the end, there before us on the blackboard were precisely, numerically, the missing colors that astronomers see in the light of distant stars.
And here’s the wonderful thing, the moral of my story: That Schrödinger, a member of the human species on planet Earth, in the neighborhood of a yellow star, one star of hundreds of billions in the Milky Way Galaxy, which is just one of tens of billions of visible galaxies in the universe, can invent a musical sort of equation that in principle — and, to a large extent, in practice — yields upon solution all of the notes of the symphony of creation.
Schrödinger, who before his passing, got out of bed, brushed his teeth, and ate breakfast just like you and me. Now if that doesn’t send shivers up your spine, you should go back to bed and forget that there’s a universe at all.