Originally published 4 June 1990
We don’t know who first imagined that the world was made of atoms.
An ancient Greek tradition attributed the idea to a Phoenician named Mochus, or Moschus. Modern scholars are more likely to give the honor to Leucippus, who lived on the shores of the Aegean Sea in the 5th century BC.
The name most of us associate with the invention of atomism is Democritus, who was a pupil of Leucippus.
Whoever first thought of it, the idea exercised a mighty hold on the human imagination for 2,500 years. Mind you, nobody ever saw an atom; they were much too small for that. Even the whisker of a flea was presumably many atoms wide. But as Socrates urged upon Cratylus, there must be something that doesn’t change or the world wouldn’t exist at all.
There is a lovely passage in De rerum natura where the Roman writer Lucretius tries to convince his readers by analogy that just because you can’t see atoms doesn’t mean they don’t exist: “For often on a hill, cropping the rich pasture, woolly sheep go creeping whither the herbage all gemmed with fresh dew tempts and invites each, and full-fed the lambs play and butt heads in fun; all which things are seen by us blurred together in the distance, as a kind of whiteness at rest on a green hill.”
Seeing what the ancients imagined
Looking back, we can only admire the cleverness of those ancient thinkers who guessed the existence of atoms. Their achievement illustrates the mysterious power of the human mind to discover the truth of things, sometimes even without the guide of direct observation.
Within recent centuries, discoveries in experimental chemistry and physics made Greek atomistic philosophy seem almost certainly correct, but it’s only in our own time that we have seen what Lucretius could only imagine — visual images of individual atoms.
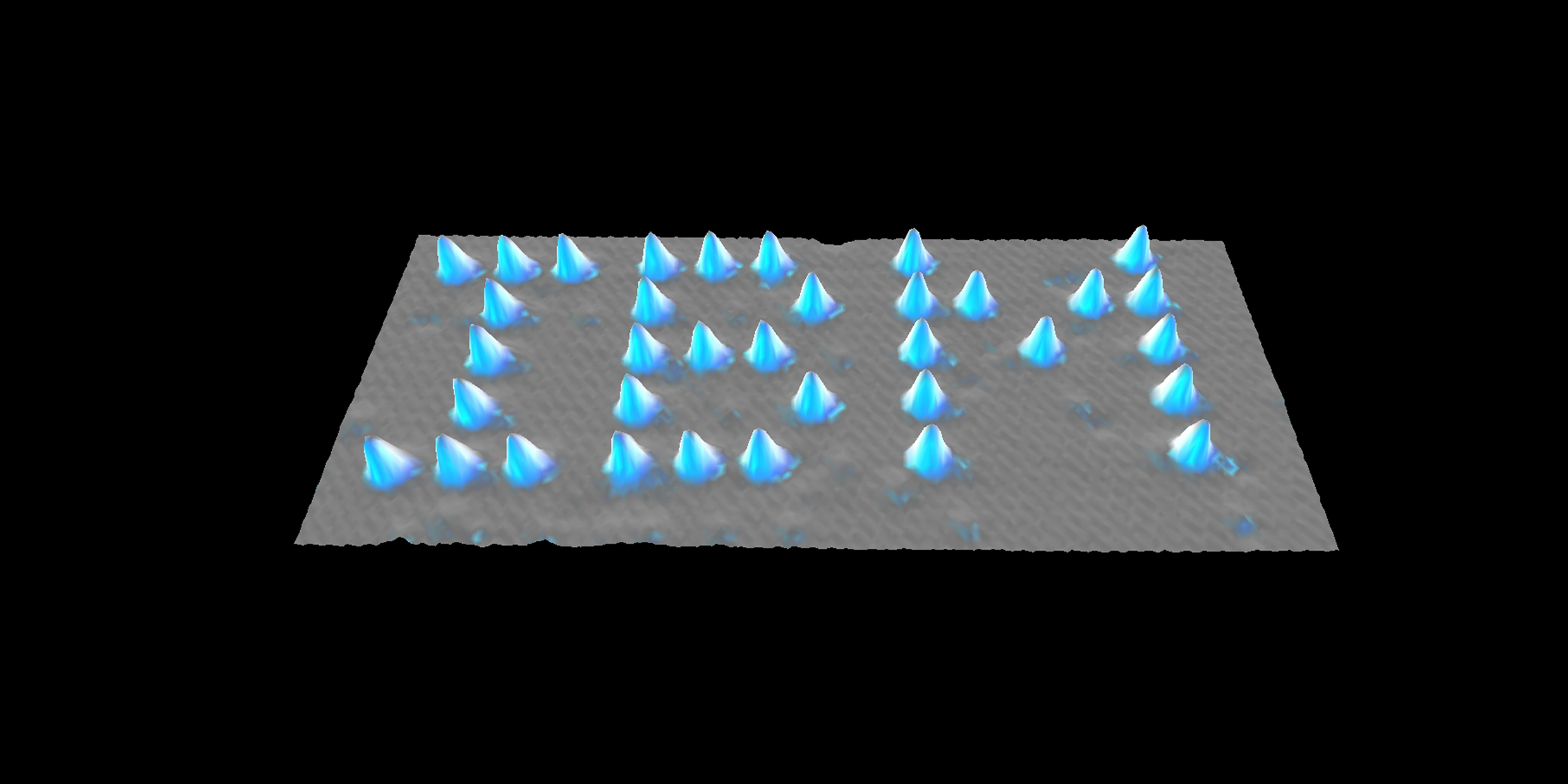
In the April 5th issue of Nature we are offered a photograph that would convince even the most obdurate skeptic of the existence of atoms: 35 fat xenon atoms lined up on a smooth nickel surface to spell IBM.
The instrument which makes possible the manipulation of individual atoms is the scanning tunneling microscope, invented in 1981 by Gerd Binnig and Heinrich Rohrer at the IBM Zurich Research Laboratory in Switzerland. The device is based on a law of quantum physics called the Uncertainty Principle.
To understand how it works, first think of a bunch of ping-pong balls rattling around in a cardboard box, with another empty box not far away. If the walls of the boxes are high enough, you will always find the balls inside box No. 1, never box No. 2. Ping-pong balls can’t pass through the walls of the boxes, only up and over — and we have assumed they don’t have enough energy for that.
Now think of the electrons in an atom as the ping-pong balls, and the force that holds the electrons in the atom as the walls of a box. But with this very big difference: The Uncertainty Principle says that we can never know the position of an electron exactly, only the probability that it will be found in a certain place. We must think of the position of an electron as a bit of a blur.
In the scanning tunneling microscope, the tip of a needle is brought very close to a material surface. If the positional blur of an electron in the tip of the needle extends across the gap, there is a chance that the electron will be found on the surface, like a ping-pong ball suddenly appearing in box No. 2 without going over the walls. The electron is said to have “tunneled” the gap.
If a voltage is applied between the tip of the needle and the surface, the transfer of electrons shows up as an electric current, and this current is exceedingly sensitive to the distance between tip and surface. As the needle is moved laterally across the surface (scanning), variations in current can be used to display an image of the surface on a video screen. Atoms show up as bumps, and the spaces between the atoms as valleys.
Lining up atoms
In the work that led to the atomic-scale IBM logo, researchers at the IBM Almaden Research Center in California used a scanning tunneling microscope to pick up and move around xenon atoms on a relatively smooth nickel surface, exploiting the tiny attractive force that exists between the tip of the needle and the atoms of the surface. Then, with the microscope in its imaging mode, the results were observed.
One-by-one, 35 xenon atoms were plucked out of a random scatter and lined up to form letters. It is not the first time atoms have been moved around with a tunneling microscope, but it is the first time that atoms have been individually arranged in a recognizable human design.
You could line up 60,000 of these logos on the head of a pin. Call it, if you want, the smallest bit of self-promotion in the history of advertising, but the atomic-scale IBM has its place in the history of ideas. With this experiment, the atomism of Leucippus completes a 2,500 year journey from inspired guess to philosophy to chemistry to physics to plain old engineering.